Background: Bruton tyrosine kinase (BTK) inhibitors (BTKi) have been shown to improve outcomes in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); however, adverse events (AEs) were the most common reason for ibrutinib and acalabrutinib discontinuation (median time ≤6 mo; Mato et al, Haematologica 2018;103:874; Yazdy et al, Blood 2019; Supplement1: 4311). Off-target effects of ibrutinib have been implicated in BTKi-related AEs. Zanubrutinib, a BTKi approved for treatment of mantle cell lymphoma (MCL) and in development for other hematologic malignancies, was specifically engineered to optimize selectivity and maximize BTK occupancy. In the head-to-head ASPEN trial of zanubrutinib vs ibrutinib in patients with Waldenström macroglobulinemia (WM), zanubrutinib showed a lower rate of AEs leading to death, discontinuation, dose reduction, and dose holds (Dimopoulos et al, EHA 2020; Abstract S225). We conducted a prospective clinical trial of zanubrutinib in patients with relapsed/refractory B-cell malignancies who have become intolerant to prior BTKi (ibrutinib and/or acalabrutinib) therapy.
Methods: In this ongoing phase 2, multicenter, US, single-arm, open-label study (NCT04116437; BGB-3111-215), the safety and efficacy of zanubrutinib monotherapy (160 mg twice daily or 320 mg once daily) is being evaluated in patients with B-cell malignancies who meet requirements for treatment and have become intolerant to prior BTKi therapy. An intolerant event was defined as an unacceptable toxicity where, in the opinion of the investigator (INV), treatment should be discontinued despite optimal supportive care as a result of 1 of the following: grade ≥2 nonhematologic toxicities for >7 days (with or without treatment), grade ≥3 nonhematologic toxicity of any duration, grade 3 neutropenia with infection or fever, or grade 4 hematologic toxicity that persists to the point that the INV chose to stop therapy due to toxicity and not disease progression (PD). All enrolled patients must not have documented PD during prior BTKi therapy. Response assessment was evaluated by INV for CLL per modified International Workshop on CLL criteria (Hallek et al, Blood 2008;131:2745; Cheson et al, J Clin Oncol 2012;30:2820), for SLL, MCL, and marginal zone lymphoma per Lugano criteria (Cheson et al, J Clin Oncol 2014;32:3059), and for WM per modified 6th International Workshop on WM criteria (Owen et al, Br J Haemtol 2013;160:171). Disease parameters (imaging and laboratory parameters) performed at study entry were used as the baseline for response assessment.
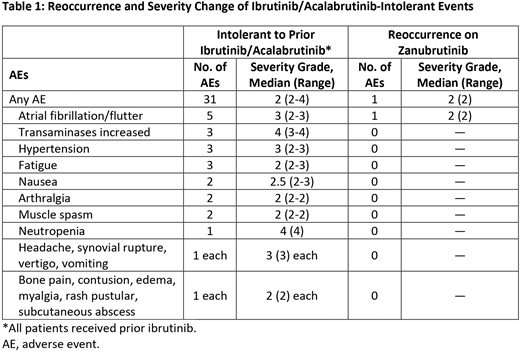
Results: As of 01 June 2020 (data cutoff), 17 patients with CLL/SLL were enrolled, received ≥1 dose of zanubrutinib, and were analyzed for safety. Median age was 70 years (range, 49-91) and median duration of treatment exposure was 3.02 mo (range, 0.56-7.59). The median number of prior regimens was 1 (range, 1-3). All patients had received ibrutinib. At data cut off, no patients had received acalabrutinib. At data cutoff, 16 patients remained on zanubrutinib treatment. One patient withdrew herself from the study following an AE (grade 3 syncope) unrelated, as per INV, to study treatment. Of the 31 BTKi-related AEs associated with intolerance (Table 1), 30 (96.8%) did not recur, and 1 event (3.2%; atrial fibrillation) recurred at a lower grade (grade 3 vs 2) and for a shorter duration (14 vs 3 days) vs the initial ibrutinib-intolerant event. Ten patients (58.8%) reported ≥1 AE. AEs reported in ≥10% of patients on zanubrutinib included dizziness (n=3; 17.6%) and cough (n=2; 11.8%). Grade ≥3 AEs were reported in 2 patients (11.8%): neutropenia and syncope (n=1 each; 5.9%). AEs of interest included hemorrhage and infections (n=3 each, 17.6%) and anemia, neutropenia, and atrial fibrillation (n=1 each; 5.9%). No AEs led to dose modification or treatment discontinuation. No serious AEs or deaths were reported. As of data cutoff, 10 patients were evaluable for efficacy with ≥1 response assessment. All 10 patients achieved at least stable disease, and 60% of these patients achieved a deepening of response since initiating zanubrutinib. Enrollment is ongoing and the presentation will include additional patients.
Conclusions: Zanubrutinib demonstrated efficacy and tolerability in CLL/SLL patients who were intolerant to previous BTKi. These data suggest that zanubrutinib may provide a potential option after intolerance to other BTKi.
Shadman:Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Atara Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellectar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG therapeutics: Research Funding; Sound Biologics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mustang Bio: Research Funding; MophoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Ended employment in the past 24 months; Sunesis: Research Funding; Gilead: Research Funding. Sharman:Celgene: Consultancy, Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding; BeiGene: Research Funding; Roche: Consultancy, Research Funding; Acerta: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding. Levy:Amgen: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Bristol Meyers Squibb: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding; Baylor University Med Center: Current Employment; Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding. Misleh:Medical Oncology Hematology Consultants (MOHC): Current Employment; High Mark Blue Cross: Membership on an entity's Board of Directors or advisory committees. Zafar:Bristol Meyers Squibb: Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); AstraZeneca: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Karyopharm: Honoraria; Sarah Canon Research Institute: Research Funding; Florida Cancer Specialists and Research Institute: Current Employment. Freeman:Summit Medical Group: Current Employment. Burke:Kura: Consultancy; Celgene: Consultancy; Gilead: Consultancy; Adaptive: Consultancy; Morphosys: Consultancy; Bristol Myers Squibb: Consultancy; Roche: Consultancy; AbbVie: Consultancy; Bayer: Consultancy; Astra Zeneca: Consultancy; Verastem: Consultancy; Epizyme: Consultancy; Seattle Genetics: Speakers Bureau; Adaptive Biotechnologies: Consultancy. Cultrera:Amgen: Speakers Bureau; Florida Cancer Specialists + Research Institute: Current Employment; Celgene: Speakers Bureau; AcroTech: Speakers Bureau; Verastem: Speakers Bureau. Yimer:BeiGene: Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau; Takeda: Speakers Bureau; Sanofi: Speakers Bureau; Epizyme: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Texas Oncology: Current Employment; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau; Karyopharm: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau; TG Therapeutics: Consultancy; Janssen: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau; Celgene, a Bristol-Myers Squibb Company: Consultancy, Membership on an entity's Board of Directors or advisory committees. Chen:BeiGene: Current Employment, Current equity holder in publicly-traded company. Zhang:BeiGene: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Cohen:BeiGene: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Ro:BeiGene: Current Employment, Current equity holder in publicly-traded company; Amgen: Current equity holder in publicly-traded company. Huang:BeiGene: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Flinn:Iksuda Therapeutics: Consultancy; Loxo: Research Funding; Kite Pharma: Consultancy, Research Funding; Karyopharm Therapeutics: Research Funding; IGM Biosciences: Research Funding; Infinity Pharmaceuticals: Research Funding; Unum Therapeutics: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; Acerta Pharma: Research Funding; Incyte: Research Funding; Janssen: Consultancy, Research Funding; Great Point Partners: Consultancy; Genentech, Inc.: Research Funding; AstraZeneca: Consultancy, Research Funding; ArQule: Research Funding; Agios: Research Funding; Takeda: Consultancy, Research Funding; Forty Seven: Research Funding; Calithera Biosciences: Research Funding; BeiGene: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Trillium Therapeutics: Research Funding; Triphase Research & Development Corp.: Research Funding; Verastem: Consultancy, Research Funding; Yingli Pharmaceuticals ≠: Consultancy, Research Funding; Rhizen Pharmaceuticals: Research Funding; Johnson & Johnson: Other; Roche: Consultancy, Research Funding; Vincera Pharma: Consultancy; Celgene: Research Funding; Merck: Research Funding; Constellation Pharmaceuticals: Research Funding; Curio Science: Consultancy; MorphoSys: Consultancy, Research Funding; Curis: Research Funding; AbbVie: Consultancy, Research Funding; Teva: Research Funding; Pfizer: Research Funding; Nurix Therapeutics: Consultancy; Novartis: Research Funding; Seattle Genetics: Consultancy, Research Funding; Portola Pharmaceuticals: Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Forma Therapeutics: Research Funding; F. Hoffmann-La Roche: Research Funding; Gilead Sciences: Consultancy, Research Funding.
Zanubrutinib has not been approved for R/R CLL/SLL, MZL, and WM in the US
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal